Fred Hutch Seeks Volunteers With COVID-19 to Join Tests of Therapies
by Sally James
In all the excitement over the new COVID-19 vaccines, it's easy to forget that the disease will strike thousands of people before the pandemic is over and we still don't have reliable treatments for the disease.
Physician Rachel Bender Ignacio wants to change that by conducting studies of four different treatment methods in Seattle — all of which are open to volunteers from the community. Bender's team calls people who have tested positive and offers them information about the studies. It is essential that people sign up within four days of receiving a nasal swab to test for the virus.
Bender Ignacio spoke to the Emerald about the research taking place at the Fred Hutchinson Cancer Research Center in Seattle.
"There is a cognitive dissonance. People don't feel well and they want to stay home, and we are asking them to come in to our clinic," she explained.
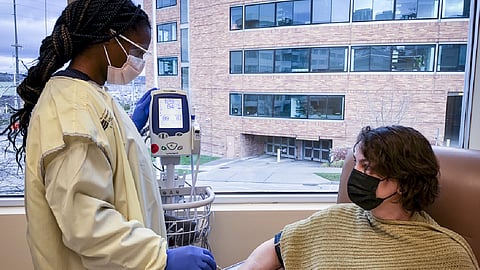
When someone visits the specialized clinic set up specifically for COVID-19 research, they can travel alone in their own car to the South Lake Union location or the clinic will send a special van to pick them up at no cost. One of the first faces they may see — behind her mask — is that of Corrie Moreau, a specialized registered nurse with additional training in giving infusions of medication and conducting clinical trials.
At the clinic, depending on which study a person signs up for, they may get a pill or medication infusion into their arm in the form of an IV drip. Half of all patients will be assigned what is called a placebo, which has no medicine in it. Researchers will test the effectiveness of a treatment by comparing people who get the medications with those who get the placebo. Neither the researchers or the patients themselves will know which patients will receive medicine and which will receive a placebo.
Moreau says her childhood as a military kid moving from place to place has helped her be outgoing and welcoming to her patients during her six years in research nursing at the Hutch. She also understands why some communities may feel more hesitant.
"As a Black person, based on history, I know why we don't have the best trust in health care," Moreau said. " But for me, one thing that drives me to be part of this study is that if People of Color are not participating, how do we really know these methods are going to be effective in our community?"
"There's always the chance that you won't get the study drug … but if you are the person who does get the drug, and we're getting information on People of Color that will help other People of Color? That would be awesome," she said.
It is part of Moreau's job and her team's work to try to make clinic visits as comfortable as possible for the volunteers. Besides free transportation, they arrange free food and snacks and there is compensation for participants. Each of the studies requires a different schedule of visits.
Clinic hours are 7 a.m. to 7 p.m. to make sure study participants can fit visits in before or after work. Moreau is one of those who stays until 7 p.m.
Bender Ignacio explains one side benefit of the study: participants receive very close, watchful attention from the researchers and team. A person may have no insurance or even a regular doctor, but if they are accepted into these studies, their health will be monitored carefully.
"When participating, you will have somebody to talk to daily," Bender Ignacio said. "You will have someone watching your vital signs and looking at you very closely, who's an ally. … And making sure that if they take a turn for the worse, we will refer them quickly to an emergency department."
Three different therapies are being studied: remdesivir, monoclonal antibodies, and molnupiravir, an antiviral that was created to treat influenza infections. News stories earlier this year reported that remdesivir shortened the hospital stays of people with COVID-19, but now its effectiveness in people who don't require hospitalization is being tested. The monoclonal antibodies from the biotech company Regeneron were in the news last year when President Donald Trump received them as part his treatment for COVID-19 while in the hospital. The Hutch is studying whether similar antibodies may benefit people who are not hospitalized.
Find information about all of the clinical trials of COVID-19 therapies currently underway at the Hutch on their website.
"We need to remember, as long as the virus is circulating, we still need treatments that work," Bender Ignacio said. The Hutch studies are part of a nationwide network of research going on in dozens of cities across the country. Results will be pooled together to reach the required number of participants to validate the findings.
Sally James is a science writer in Seattle. You can read more of her work at www.seattlesciencewriter.com. She's written about biotech, cancer research, and health literacy and volunteered as president of the nonprofit Northwest Science Writers Association.
Featured Image: A clinical study participant and a member of the study staff at the COVID-19 Clinical Research Center at Fred Hutch's campus in Seattle. Photo courtesy of Fred Hutchinson Cancer Research Center.
Help keep BIPOC-led, community-powered journalism free — become a Rainmaker today.